Drawback and Reconciliation Updates for ACE – CBP Announces Deadlines
Mark your calendars! Customs and Border Protection (CBP) announced July 8, 2017 as the effective date for transition to make the Automated Commercial Environment (ACE) the sole electronic data interchange (EDI) system authorized by the Commissioner of CBP for processing electronic drawback, duty deferral entry and entry summary filings. After the effective date, the Automated Commercial System (ACS) will no […]
Continue Reading »Food Importers Face FSVP Deadline, May 30, 2017
Attention food importers! The May 30 deadline to comply with FDA’s Foreign Supplier Verification Programs (FSVP) is mandatory and right around the corner. The Food Supplier Verification Program (FSVP) rule is intended to be a flexible, risk-based program to verify that foreign suppliers are producing their food in compliance with processes that meet the FDA’s […]
Continue Reading »New Executive Order Targets AD/CVD Violations
U.S. Customs and Border Protection will begin to implement “Establishing Enhanced Collection and Enforcement of Antidumping and Countervailing Duties and Violations of Trade and Customs Laws.” The Executive Order, signed on March 31, promotes the efficient and effective administration of U.S. Customs and trade laws by establishing enhanced measures to collect duties and a heightened enforcement […]
Continue Reading »TSCA Blanket Certification Deadline Announced
U.S. Customs and Border Protection (CBP) issued a final rule regarding the importation of Chemical Substances and Mixtures Subject to EPA’s Toxic Substance Control Act (TSCA), eliminating the old TSCA Blanket Certification process. While the original effective date was temporarily postponed, CBP has announced an implementation deadline of March 20, 2017. The new ruling will […]
Continue Reading »FDA Currently Experiencing Multiple System Outages
FDA is currently experiencing outages across many applications, which prevents the processing of entries. The technical team is currently investigating. As a result, FDA’s Division of Food Defense Targeting will be operating under the prior notice scenario 2 contingency. These issues will prevent submitters who file via Automated Broker Interface (ABI) from receiving Prior Notice […]
Continue Reading »Attention TSCA Importers – Blanket Certification Update Required!
As the new year comes in, so do new regulations form U.S. Customs and Border Protection (CBP) and the Environmental Protection Agency (EPA) regarding the importation of Chemical Substances and Mixtures Subject to EPA’s Toxic Substance Control Act (TSCA). CBP issued a final rule in the Federal Register on December 27, 2016 eliminating the old TSCA […]
Continue Reading »CBP Releases Guidance for Inbound Hanjin Vessels
Scenario 1: Vessel Diverted to Foreign Port and Discharged: A Hanjin vessel does not arrive in the intended U.S. port and diverts to a foreign port to discharge freight. The manifest and Importer Security Filing (ISF) must be deleted. All bills of lading need to be deleted (not cancelled) Entries and entry summaries need to […]
Continue Reading »Urgent Notice! Importer Security Filing (ISF) Enforcement Update
Customs & Border Protection (CBP) at U.S. ports is no longer required to send requests for liquidated damages (LD) to its Headquarters for approval. Non-compliant Importer Security Filings can result in cargo holds at the port instead of, or in addition to, liquidated damage claims. Additionally, liquidated damage claims against importer bonds will no longer […]
Continue Reading »Newly Required FDA Cosmetic, Food and Medical Device Data Elements in ACE
Shapiro is fully programmed for all FDA filings and has been actively filing FDA though ACE for many months. Under the ACE platform FDA is requiring additional, and in many cases new information, to be submitted in import entries. Specifically all entries for FDA Food, Cosmetics and Medical Devices are affected. Below is a list […]
Continue Reading »CBP Sets An Ambitious Deadline for FDA Entries and Entry Summaries of June 15, 2016
As of June 16, CBP will require filing in ACE of FDA entries and entry summaries under entry types 01, 03, 06, 11, 23, 51 and 52. CBP boldly stated that the Automated Commercial System (ACS) “will no longer be a CBP-authorized [electronic system] for purposes of processing these electronic filings.” While the agency concedes […]
Continue Reading »Obama Signs the Trade Facilitation and Trade Enforcement Act into Law
On Wednesday, February 24, 2016, President Obama signed into law the Trade Facilitation and Trade Enforcement Act of 2015 (H.R. 644). The comprehensive authorization is the first overhaul of the United States Customs and Border Protection Agency (CBP) since 2003. The bipartisan legislation seeks to strengthen trade enforcement at ports, to fortify detection of duty […]
Continue Reading »Customs Reauthorization Bill Heads to Obama
On Thursday, February 11th, the U.S. Senate passed the Trade Facilitation and Trade Enforcement Act of 2015 (H.R. 644), the first overhaul of the United States’ Customs and Border Protection Agency in over 15 years. The legislation will give teeth to programs protecting U.S. businesses from unfair trade practices and rampant ‘trade cheats.’ The bill […]
Continue Reading »Technical Problems with FDA Processing Through ACE
With the ACE February 28th deadline looming, many filers are reporting various technical problems involving FDA processing that are resulting in extreme issues across the board. To outline some examples: Messages are not returning to the filer’s software at all. The FDA Review is received, but there is no follow up message Rejected FDA entries […]
Continue Reading »How Exporters Can ACE AESDirect!
How will ACE affect AES Direct Portal for exporter? Within the coming few months, the AESDirect Portal will go live in the Automated Commercial Environment (ACE). All exporters must have an ACE account to access the new AESDirect system, to be known as Refactored AESDirect. Accounts will not transition from the existing system. The existing […]
Continue Reading »Are You Ready for ACE Compliance?
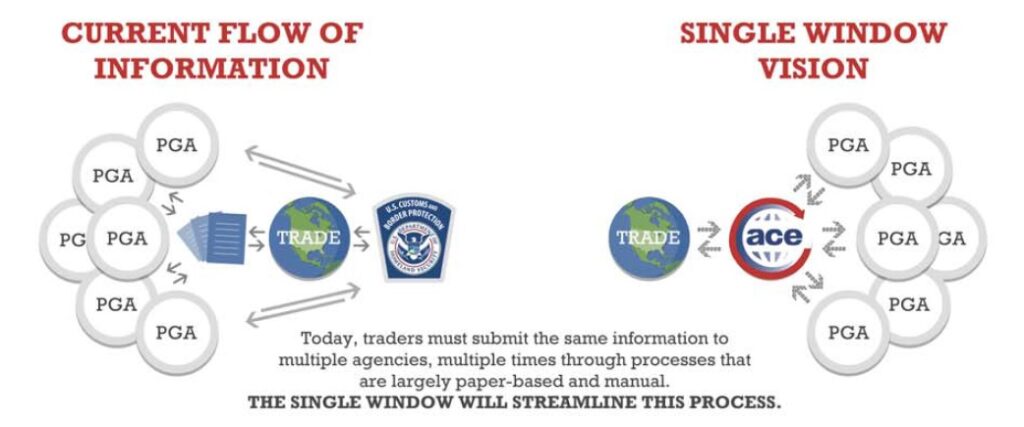
Check out our ACE FAQ guide to help answer your import and export questions! What is ACE? The Automated Commercial Environment (ACE) is the primary system or “single window” for the international trade community to submit import and export data to communicate with U.S. Customs and Border Protection (CBP) and other participating government agencies. CBP and […]
Continue Reading »Trans-Pacific Partnership Members Reach Trade Deal
Ministers of the 12 Trans-Pacific Partnership (TPP) countries concluded trading negotiations on October 4th, completing one of the most ambitious multilateral trade deals of the last two decades and paving the way for lower tariffs and increased trade standardization throughout the Pacific Asia region. While the complete details of the 30 chapter agreement have not […]
Continue Reading »GSP Gets First Round of Edits
While the Office of the US Trade Representative continues its ongoing review of the newly reauthorized Generalized System of Preferences (GSP), President Obama has issued a proclamation with the first round of revisions. Countries Removed: Seychelles, Uruguay and Venezuela have become high-income countries as defined by the official statistics of the World Bank and will […]
Continue Reading »Automated Commercial Environment (ACE) Deadline Postponed
The President’s order “Streamlining the Export/Import Process for America’s Businesses”, dictates an electronic information exchange capability, or “single window” through which businesses will transmit data required for the importation or exportation of cargo – the Automated Commercial Environment (ACE). The Executive Order requirements by December 31, 2016: Transmission of a harmonized set […]
Continue Reading »Filers Now Able to File GSP-Eligible Entry Summaries
US Customs and Border Protection has just announced that, effective July 29, 2015, filers again are entitled to file GSP- eligible entry summaries without the payment of estimated duties. Please be aware that the GSP reauthorization provides retroactive benefits only to goods from a country that is a beneficiary of the GSP program as of […]
Continue Reading »GSP Renewal Clarification
As you are aware, the president recently signed off on the Trade Preferences Extension Act which retroactively renews GSP. At this point and time, all that has changed is learning that this legislation both extends GSP and retroactively applies benefits to multiple commodities through the end of 2017. Instructions regarding refunds and the process to […]
Continue Reading »